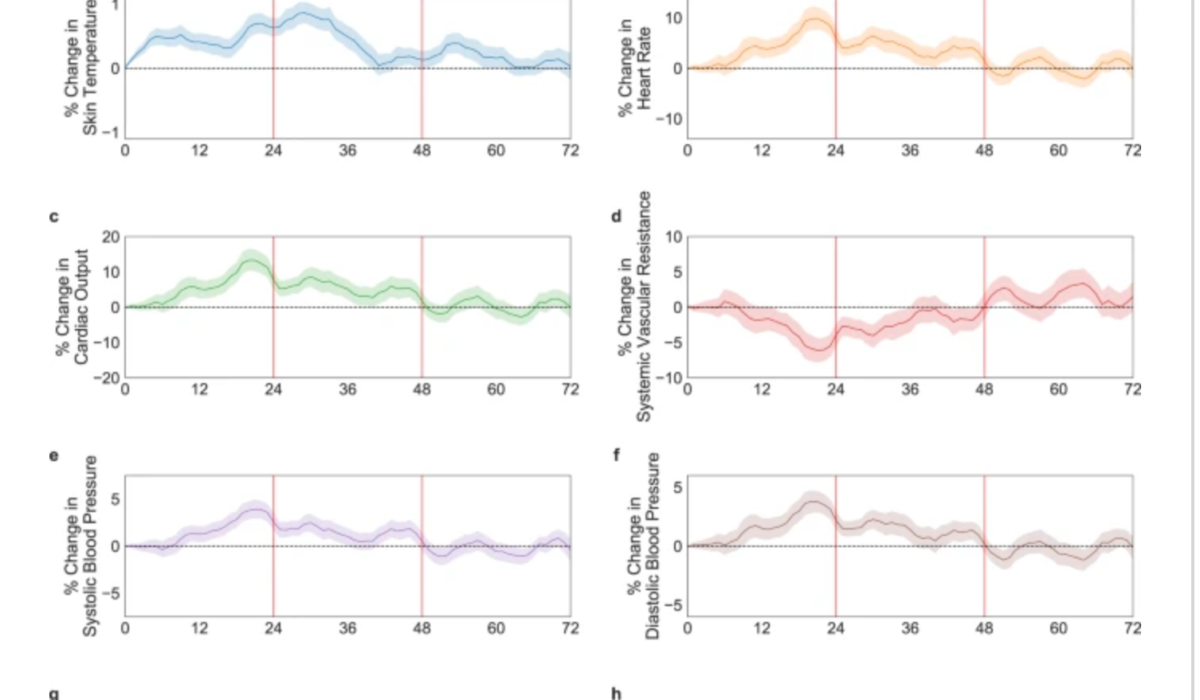
Wearable sensors could improve clinical trials by enabling earlier identification of abnormal reactions. Currently, vaccine safety in clinical trials is primarily determined by participants’ subjective self-reporting.
Dan Yamin, Yiftach Gepner, and Tel Aviv University colleagues used a chest patch sensor to monitor various health indicators in 160 participants, before and after receiving the Pfizer BioNTech COVID 19 vaccine. Participants also self-reported using a mobile phone app.
Significant changes in health indicators following vaccine administration were detected by the chest patch sensor in participants who did and did not report changes. Three days following vaccination, participant health indicators returned to the levels observed the day before vaccination in both groups.
Click to view Tel Aviv University video